No. S 479
| Sale of Food Act |
(Chapter 283)
| Food (Amendment No. 2) Regulations 1998 |
|
| Citation and commencement |
| 1. These Regulations may be cited as the Food (Amendment No. 2) Regulations 1998 and shall come into operation on 15th September 1998. |
| Amendment of regulation 11 |
2. Table 1 of the regulation 11 of the Food Regulations (Rg 1) is amended by inserting, immediately below the item “Vitamin B6, pyridoxine, pyridoxal, pyridoxamine”, the following items and entries in the appropriate columns as shown hereunder:
|
| Amendment of regulation 18 |
| Amendment of regulation 22 |
| 4. Regulation 22(2) of the Food Regulations is amended by inserting, immediately after the words “propylene glycol” in the fifth line, the word “, triacetin”. |
| Amendment of regulation 31 |
5. Regulation 31 of the Food Regulations is amended —
|
| New regulation 91A |
6. The Food Regulations are amended by inserting, immediately after regulation 91, the following regulation:
|
| Amendment of regulation 117 |
7. The Food Regulations are amended by renumbering regulation 117 as paragraph (1) of that regulation, and by inserting immediately thereafter the following paragraphs:
|
| Amendment of regulation 252 |
8. Regulation 252 of the Food Regulations is amended by inserting, immediately after paragraph (8), the following paragraph:
|
| Amendment of Fifth Schedule |
9. The Fifth Schedule to the Food Regulations is amended by inserting, immediately below the item “Drinking chocolate concentrate”, the following item and entry in the appropriate columns as shown hereunder:
|
| Amendmend of Sixth Schedule |
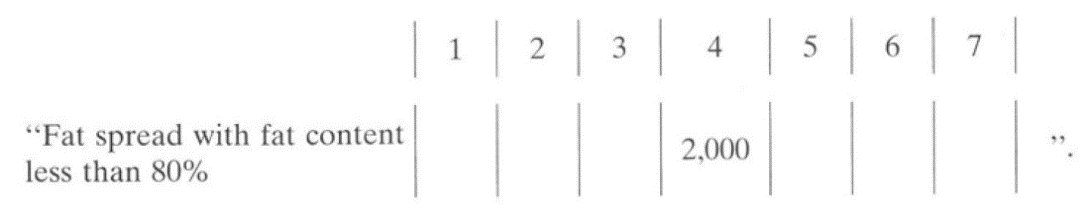
10. Part I of the Sixth Schedule to the Food Regulations is amended by inserting, immediately below the heading “Green Shade” in item 3, the following item in the appropriate columns:
|
| Amendment of Seventh Schedule |
| 11. The Seventh Schedule to the Food Regulations is amended by inserting, immediately after the words “carboxyl methyl cellulose;” in the item relating to “Cellulose”, the words “croscarmellose sodium;”. |
| Amendment of Ninth Schedule |
12. The Ninth Schedule to the Food Regulations is amended —
|
[G.N. No. S 491/91; S 179/92; S 238/92; S 336/92; S 398/93; S 340/98]
Permanent Secretary, Ministry of the Environment, Singapore. |
| [ENV/LD/CF/39 Vol. 3 (7); AG/LEG/SL/283/98/1 Vol. 1] |
| (To be presented to Parliament under section 38(3) of the Sale of Food Act). |