No. S 384
| Medicines Act |
(Chapter 176)
| Medicines (Licensing, Standard Provisions and Fees) (Amendment) Regulations 2004 |
|
| Citation and commencement |
| 1. These Regulations may be cited as the Medicines (Licensing, Standard Provisions and Fees) (Amendment) Regulations 2004 and shall come into operation on 1st July 2004. |
| New regulation 5B |
| New Sixth Schedule |
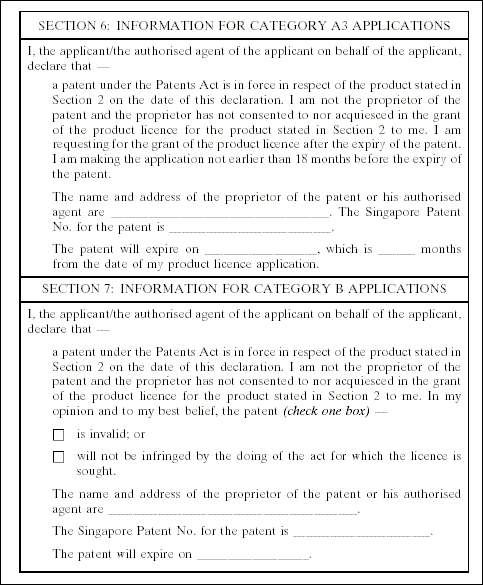
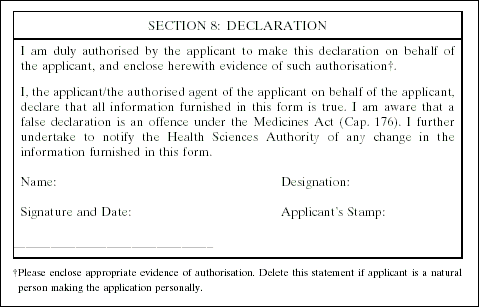
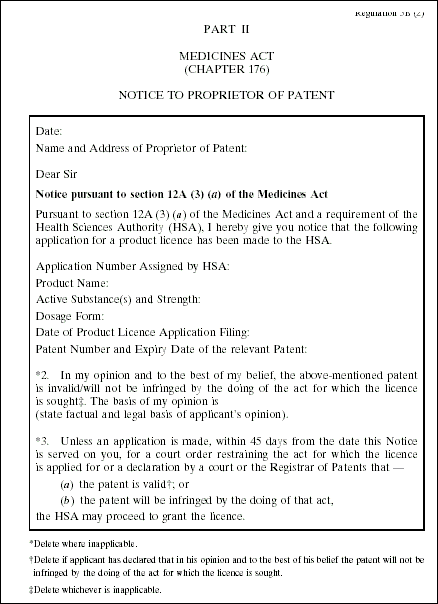
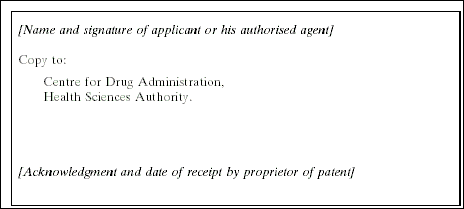
3. The Medicines (Licensing, Standard Provisions and Fees) Regulations are amended by inserting, immediately after the Fifth Schedule, the following Schedule:
[G.N. Nos. S 309/2001; S 641/2002; S 621/2003] |
Made this 29th day of June 2004.
Permanent Secretary, Ministry of Health, Singapore. |
| [HSA(CDA) 78:09; AG/LEG/SL/176/2002/1 Vol. 2] |