No. S 439
| Human Organ Transplant Act |
(Chapter 131A)
| Human Organ Transplant (Amendment No. 2) Regulations 2009 |
|
| Citation and commencement |
| 1. These Regulations may be cited as the Human Organ Transplant (Amendment No. 2) Regulations 2009 and shall come into operation on 1st November 2009. |
| Amendment of regulation 4 |
| New regulations 6A and 6B |
3. The principal Regulations are amended by inserting, immediately after regulation 6, the following regulations:
|
| Amendment of Second Schedule |
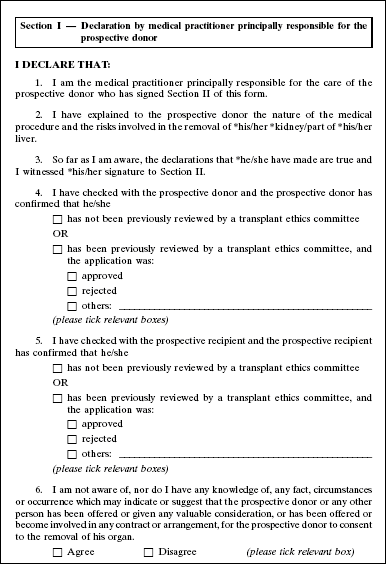
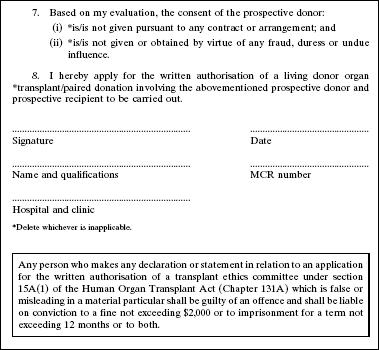
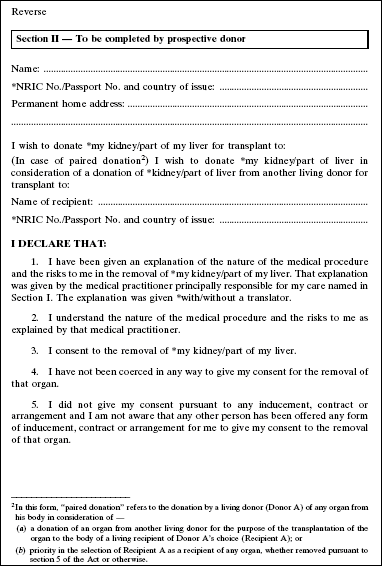
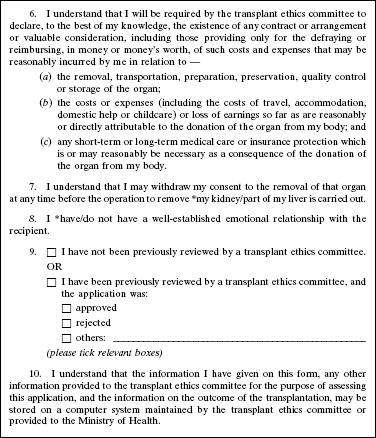
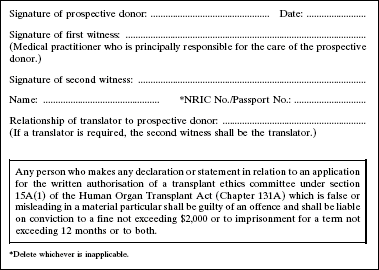
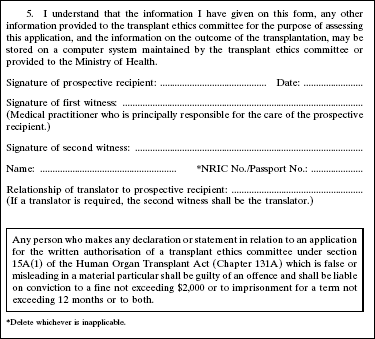
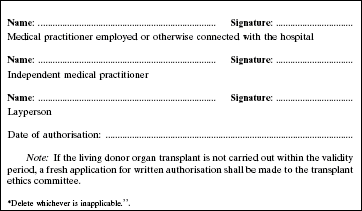
4. The Second Schedule to the principal Regulations is amended by deleting Forms 1 and 2 and substituting the following Forms:
[G.N. Nos. S 380/2008; S 309/2009] |
Made this 23rd day of September 2009.
Permanent Secretary, Ministry of Health, Singapore. |
| [MH 078:018/005-000 V007; AG/LEG/SL/131A/2003/1 Vol. 2] |